‘Visceral silence‘ makes room for mental food noise – this is one the paradoxes of obesity, which could be described as Pandora’s box. In Greek mythology Pandora was given a jar (later called as a “box”). When she opened it, all the evils of the world escaped, leaving only Hope inside once she closed it again. In obesity, every meal becomes a Pandora’s box – once opened, appetite floods out, and when closed, only shame remains.
When the Energy Balance Box Doesn’t Fit: Out-of-the-Box Thinking for Obesity.
The energy imbalance simplification is wrong. Explaining obesity pathogenesis solely through the energy balance lens is a reductionist view.
Plenty of publications have been written here on this topic, yet the final drop still hasn’t fallen to sink the energy imbalance theory of obesity.
So what’s wrong with the energy balance framework?
1️⃣ Energy balance can remain unimpaired in someone with a high BMI and significant adiposity.
2️⃣ Obesity and chronic overeating aren’t linked by a simple cause-and-effect logic. Many experts now argue that chronic overeating is a consequence of obesity.
Example: “Overeating does not cause obesity; obesity causes overeating. Undereating does not fix obesity; fixing obesity leads to undereating” Dr. Kushner at YWM2025.
It’s a bidirectional link -> a vicious cycle.
3️⃣ Chronic overeating may result from an absolute or relative deficiency of nutrient-sensing signals from peripheral organs (not just the gut) to the brain. This dysfunction can start in bodies of normal shape, composition, and BMI — and apparently lead to various diseases. And here’s where you might raise an eyebrow: this same CNS-level glitch can drive two seemingly opposite conditions — obesity through chronic overeating, or cachexia through anorexia nervosa and chronic undereating – both diseases are associated with hyperactivation of AgRP neurons [1-4].
4️⃣ Chronic overeating isn’t natural — not for humans, not for animals. Yes, our ancestors might have eaten to maximum stomach capacity when food was scarce and suddenly abundant — but they didn’t eat themselves to death. Animals won’t either — unless you artificially push them there. If you stimulate AgRP neurons non-stop and provide endless food, yes, they might gorge to death [5-7]. But is that natural behaviour?
5️⃣ Question: What in our modern environment acts like chronic overstimulation of AgRP neurons? And why does overstimulated AgRP activity push one person towards uncontrollable appetite and overeating, while in another it triggers “nervosity” and a complete loss of appetite — anorexia nervosa (AN)? If there’s anything worth weighing on the scale, it’s NOT energy intake and energy expenditure, it’s the regulatory circuits between AgRP and POMC neurons in the brain.
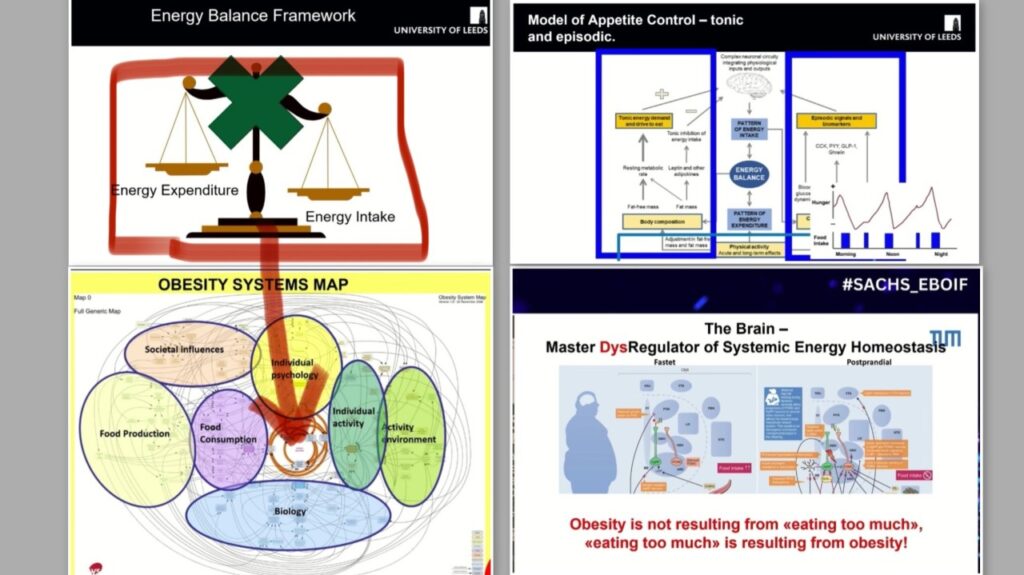
Some of the most insightful talks I’ve heard on this were by Prof. John Blundell and Prof. Katharina Timper at the Sachs 1st European Biopharma Obesity Innovation Forum. Take a look at a cover collage of few slides — they shine a light on this topic from a very different angle than “eat less, move more.”
Trying to squeeze the complexity of obesity into that tiny box on the obesity atlas — labelled core engine: energy balance — is misleading at best. And just because this box sits at the centre of the obesity atlas doesn’t mean the cause of obesity is rooted there. As noted above, the balance of energy intake and expenditure may not even be disturbed for a long time while someone carries extra kilos. Storing excess energy in fat tissue is, in fact, normal.
This energy balance framework fails to explain (to name just a few):
· The imbalance of incretins and their impaired production along the entire gut
· Deficient signaling to the brain about excessive intake/storage and, consequently, dysregulated appetite inhibition
· Impaired inter-organ crosstalk due to poor nutrient sensing in the gut
· Overexpression of obesogenic genes
· Adipose tissue that fails to adapt to excess calories, including impaired adaptive thermogenesis
· Epigenetic memory of obesity that is retained in adipose tissue even after weight loss [8]
· Systemic inflammation fueled by visceral adipose tissue
· Overstimulated AgRP neurons and a deficit of inhibitory signals from POMC neurons that regulate appetite, insulin sensitivity, and energy expenditure
Pandora’s box of Obesity
Model of appetite control – tonic and episodic.
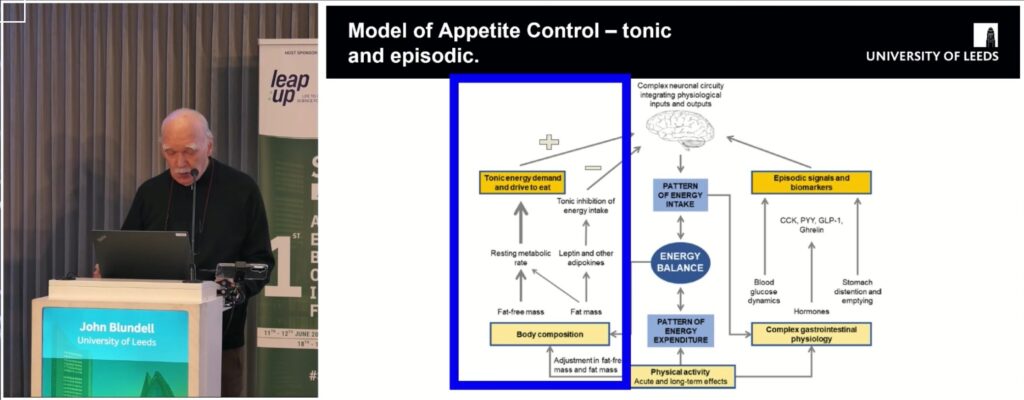
I looked at the model of tonic and episodic appetite control from another angle when I thought about where GLP-1 is actually produced.
First, GLP-1 comes from pancreatic alpha cells in response to neural stimulation — the anticipatory GI response to the sensory ‘priming’ signals of food (the classic conditional phase of Pavlov’s reflex). This pancreas-derived GLP-1 could contribute to inhibition of episodic appetite, activated by neural (hedonic) triggers. Short loop of episodic appetite control would be closed.
Meanwhile, the main source of GLP-1 is the L-cells in the ileum and colon — which likely depend heavily on what fills this part of the gut: chyme with fibers and microbiome. This tunes tonic appetite — the drive to eat, which may be equivalent of homeostatic appetite regulation. So perhaps that’s why, despite high body fat mass, there’s not enough braking of the tonic drive to eat: the signal from the lower gut is missing – the ileal brake is broken.
And it’s not just about GLP-1. There are dozens of other nutrient-sensing gut hormones and signaling pathways that shape the brain – and then the body – in how we eat, store and spend energy…
Prof. Brüning gave an excellent presentation at ECO2025 showing how sensory food perception controls melacortin neurons. Two of key conclusions fitting the tonic and episodic model of appetite:
- Melacortin (AgRP/POMC) neurons coordinate the adaptation of integrative physiology to the energy state of the organism
- In addition to homeostatic, hormonal feedback regulation, they integrate sensory information to prime anticipatory physiology responses.
Obesity reveals a biological Pandora’s box – neuronal excitation emerges rapidly with eating (and imagining food), but the counterbalancing visceral signals fail to arrive, producing persistent hunger. Each time a person opens their mouth to eat, a cascade of excitatory brain signals is released, triggering appetite. Yet the gut’s balancing signals, meant to close the loop, are delayed or absent – a state I call visceral silence, some people call it a visceral void, as if the stomach were bottomless. Without this closed-loop regulation, the neural drive to eat grows and persists – all evils of the world released. And when the person finally closes their mouth, what remains inside their own “Pandora’ box” in not hope, but shame, which triggers other vicious cycles.

Links to other valuable thoughts on this topic:
References:
- Marcelo O. Dietrich, Marcelo R. Zimmer, Jeremy Bober, Tamas L. Horvath, Hypothalamic Agrp Neurons Drive Stereotypic Behaviors beyond Feeding, Cell, Volume 160, Issue 6, 2015, Pages 1222-1232, ISSN 0092-8674, https://doi.org/10.1016/j.cell.2015.02.024.
2. GABAergic disinhibition from the BNST to PNOCARC neurons promotes HFD-induced hyperphagia Sotelo-Hitschfeld, Tamara et al. Cell Reports, Volume 43, Issue 6, 114343, June 25, 2024
3. Comprehensive insights into emerging advances in the Neurobiology of anorexia. Mao, Lian Wang, Zhihai Huang, Jian-Kang Chen, Lorelei Tucker, Quanguang Zhang, Journal of Advanced Research, 2025, ISSN 2090-1232, https://doi.org/10.1016/j.jare.2025.03.046.
4. Kriebs, A. Long-lasting effects of obesity on appetite neurons. Nat Rev Endocrinol 16, 538 (2020). https://doi.org/10.1038/s41574-020-00407-8
5. AgRP neurons: Regulators of feeding, energy expenditure, and behavior. Jennifer D. Deem, Chelsea L. Faber, Gregory J. Morton. Volume289, Issue Special Issue: Neurobiology, April 2022. https://doi.org/10.1111/febs.16176
6. Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011 Mar;14(3):351-5. doi: 10.1038/nn.2739. Epub 2010 Jan 5. PMID: 21209617; PMCID: PMC3049940.
7. Kerem Catalbas, Tanya Pattnaik, Samuel Congdon, Christina Nelson, Lara C. Villano, Patrick Sweeney, Hypothalamic AgRP neurons regulate the hyperphagia of lactation, Molecular Metabolism, Volume 86, 2024, 101975, ISSN 2212-8778. https://doi.org/10.1016/j.molmet.2024.101975.
8. Hinte, L.C., Castellano-Castillo, D., Ghosh, A. et al. Adipose tissue retains an epigenetic memory of obesity after weight loss. Nature 636, 457–465 (2024). https://doi.org/10.1038/s41586-024-08165-7
Leave a Reply